Impact of minerals in water on dairy cows
Water is the most essential of all nutrients required by animals. Water functions in the digestion and metabolism of nutrients, elimination of waste products from the body via urine, feces and respiration, transport of nutrients and other compounds into and out of cells, electrolyte balance in the body and as a fluid environment for the developing fetus. A lactating dairy cow has one of the largest requirements for water of any animal. This is because 56 to 81% of her body weight is water and she needs to replace the major loss of water through milk production, milk is 87% water, each day (Murphy, 1992). Therefore, it is essential dairy cattle consume adequate quantities of water each day to meet their requirements.
Drinking water is the primary source and method of meeting the daily water requirement with the water contained in feed making a small contribution towards the daily requirement. On average, a lactating dairy cow will drink 95 liters of water each day. Therefore, providing dairy cattle an adequate supply of clean palatable water is important.

Because water is an excellent solvent, it often contains many different elements and compounds in addition to hydrogen and oxygen (H2O). Quality of water is a vague term but usually defined by one or more of the following characteristics: odor, taste, appearance, physical and chemical properties, macro- and micro-mineral content, presence of toxic substances and microbial contamination.
We know these characteristics affect the palatability and consumption of water by humans, but whether they have a similar affect on water consumption of animals is not well known. Certain minerals like nitrates and sulfates along with microbial contamination and many organic compounds in water are known to affect the health of both humans and animals. However, the effect of high concentrations of most macro- and micro-minerals in water on water intake, health and performance of dairy cattle is relatively unknown. A thorough review of water quality was conducted by Beede (2005).
This paper looks at the impact minerals in water has on dairy cattle production.
Water Intake
Equations to predict the water consumption of lactating dairy cattle have been developed by Castle and Thomas (1975), Little and Shaw (1978), Murphy et al. (1983), Stockdale and King (1983), Holter and Urban (1992), and Dahlborn, et al. (1998). The major factors influencing water intake are: dry matter (DM) intake, ingredient composition of the diet, percent DM of the diet, milk production, environmental conditions and nutrient content of the diet, particularly sodium, salt and protein. The Dairy NRC 2001 suggests using the Murphy et al. (1983) equation to predict water intake of lactating dairy cows as it includes many of the major factors affecting water intake.
Drinking water intake (kg/d) = 15.99 + (1.58 x DMI, kg/d) + (0.9 x milk, kg/d) + (0.05 x Na intake, g/d) + (1.20 x min temp C) (Murphy et al., 1983)
Research on drinking water intake of dry cows is limited. Holter and Urban (1992) identified dietary variables of DM and crude protein (CP) in the following equation as factors affecting water intake of dry cows:
Free water intake (kg/d) = -10.34 + (0.2296 x dry matter % of diet) + 0.2212 x DMI (kg/d) + (0.03944 x (CP% of diet)2) (Holter and Urban, 1992)
Cows only spend about 12 to 15 minutes per day drinking water. The highest water intake periods are immediately following milking and during feed consumption. Dado and Allen (1995) reported cows in tie-stalls drank an average of 13 to 15 times per day and consumed between 5 and 7 liters per drink for a drinking rate of about 4 liters per minute. Estimates of water intake for cows in loose housing are 11 to 19 liters per minute from troughs (McFarland, 1998).
A frequent comment on dairy farms where water quality problems are suspected is: cows are not drinking enough water. Cows will consume water in direct proportion to their physiological needs (milk production, maintenance, growth and gestation) and DM intake. There is no evidence to suggest cows will luxury consume water above their needs or that increasing water consumption beyond requirements will stimulate DM intake and/or milk production. Thus, the challenge in the diagnosis of dairy farm production problems is determining if milk production is being limited by the quantity and/or quality of water consumed or if milk production is limited by other factors and cows are drinking to meet milk production requirements.
Water Quality Criteria
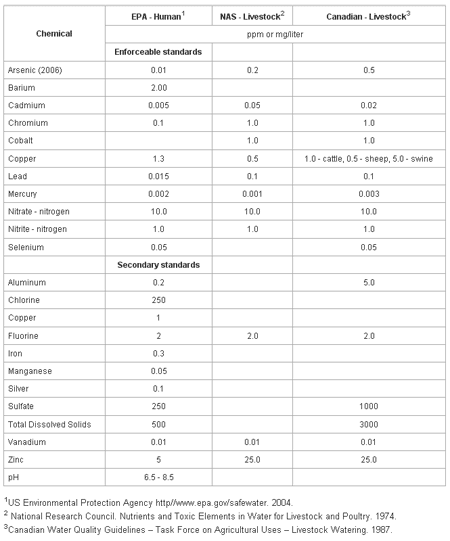
The macro- and micro-mineral concentrations in water included in the drinking water standards for humans (United States) and animals (United States and Canada) are in Table 1. The standards are divided into two categories, enforceable and secondary. The enforceable standards are levels which can not be exceeded in safe drinking waters and if exceeded, action must be taken to reduce the concentration. Secondary standards are levels at which cosmetic (tooth or/and skin discoloration) or aesthetic (taste, smell, color) effects are apparent, but no action is required to reduce the concentration.
The following measures or minerals in water are used to assess the quality of water. Their known impact through research documentation on animal health or performance is discussed. Most all quality related measures will be reported in ppm (parts per million) which is equivalent to mg/l (milligrams per liter).
Total Dissolved Solids (TDS), Total Soluble Salts (TSS) or Salinity.
Total dissolved solids, total soluble salts and salinity are physiochemical properties of water used to assess water quality. These terms are used synonymously and measure the amount of total inorganic matter dissolved in water which includes: sodium, chloride, bicarbonate, sulfate, calcium, magnesium, silica, iron, nitrate, strontium, potassium, carbonate, phosphorus, boron and fluoride in water. Because TDS is a sum of inorganic minerals, the actual mineral composition of TDS waters can be quite different. Saline or NaCl water is one of the more common causes of high TDS water, but the affect on water intake and animal performance is likely to be less than when the same TDS level is a result of high sulfate combined with magnesium and/or sodium. Research to determine the effects of TDS on the performance of lactating dairy cattle has produced varying results on water intake, feed intake and milk production. When TDS levels in water are less than 3,000 ppm, there is little to no affect on cattle, although at first introduction there may temporarily be a mild case of diarrhea. Between 3,000 and 5,000 ppm TDS, the effects on milk production and animal performance are variable, however, high TDS water is more likely to decrease milk production during summer months than in winter months (Solomon et al., 1995; Sanchez et al., 1994; Challis et al., 1987; Jaster et al., 1978). The TDS guidelines suggest water containing <5,000 ppm TDS may be fed to lactating cattle, but water containing >7,000 ppm is unacceptable for all cattle (NRC, 2001).
Hardness.
Hardness, also a physiochemical property of water, is generally a measure of calcium and magnesium ions in water. Zinc, iron, strontium, aluminum, and manganese can also contribute to water hardness; however, they are generally present in very low concentrations. Water is classified as soft at 0-60 ppm, moderately hard at 61-120 ppm, hard at 121-180 ppm and very hard at >180 ppm. Water intake of cattle and milk production were unaffected by water containing up to 290 ppm of hardness (NRC, 1974).
Nitrates.
Drinking water, especially from surface or shallow ground water, may become contaminated with high levels of nitrates. Nitrate poisoning results from a bacterial reduction of nitrate to nitrite in the rumen with the nitrite being absorbed into the blood and reducing oxygen carrying capacity. Production and reproduction were unaffected in dairy cattle consuming water containing 86 ppm nitrate-nitrogen for almost two years in a Wisconsin study (Kahler, et al., 1974), but some reproductive performance decline (increased services per conception and longer calving interval) was noted in the third year. A more recent field study in Iowa (Ensley, 2000) found a slight decline in reproductive performance in herds where nitrate-nitrogen levels were highest (approximately above 20 ppm nitrate-nitrogen). Concentrations of less than 10 ppm nitrate-nitrogen or 44 ppm of nitrate concentration in water are considered safe for dairy cattle (NRC, 2001).
Sulfates.
Calcium, iron, magnesium and sodium salts are common forms of sulfate found in water. High concentrations of sulfates, especially sodium sulfate, produce a laxative effect in cattle, but normally within a short period of time cattle become acclimated to the water and diarrhea is no longer apparent. Sulfate concentrations <500 ppm and <1,000 ppm are generally recommended for calves and adult cattle, respectively. However, the effects on animal performance and the concentration of sulfate in the water where animal performance is noticed is variable and dependent on the specific form of sulfate in the water.
Hydrogen sulfide is less common in water, but the most toxic form. Concentrations over about 0.1 ppm produce rotten egg smell.
High sulfate water can have a deleterious effect on cattle performance. Cattle consuming water with a sulfate concentration >3,500 ppm had decreased feed and water intake (Weeth, et al., 1971). However, no effect on feed and water intake or growth was seen when cattle consumed water up to 2,500 ppm of sulfate for 90 days (Digesti, et al., 1976). Recent research by Loneragan et al. (2001) has shown a linear decrease in average daily gain of feedlot steers as sulfate concentration in the drinking water increased from 136 to 2,360 ppm. Over the 16-week study, cattle initially consuming 290 and 590 ppm sulfate water had the fastest gains, but towards the end of the study higher sulfate waters seemed to improve gains. In general, water sulfate concentrations >583 ppm, equivalent to 0.22% of the diet DM, decreased feedlot cattle performance.
Table 1. Drinking water standards for humans and livestock.
pH.
A guideline for pH of water has not been established due to a lack of research, but some have suggested ranges between 6.0 and 9.0 are acceptable. It is generally assumed water between 6.0 and 8.5, human pH guideline, is satisfactory for dairy cattle.
Iron.
A high concentration (>0.3 ppm) of iron in drinking water is common. Human information suggests a high concentration of iron in water may reduce palatability and therefore consumption of water. Excess intakes of iron have been found to affect health through increasing reactive oxygen species (oxidative stress) which damage cell membranes and interrupt several biochemical reactions in the body. Oxidative stress in dairy cows has been related to increased incidences of mastitis, retained fetal membranes, and a general decrease in immune function. The aesthetic problem with iron is in the presence of iron-loving bacteria; reddish to black stains and slime form, reducing water intake, staining porcelain, and eventually restricting water flow through pipes.
The chemical form of iron in water is an important determinant of the bioavailability of iron and how oxidative reactive it may be. Most of the iron in water is soluble and found as ferrous (Fe++). However, the solubility and form of iron will change with pH and sulfate content of the water. At a pH of less than 7, more of the iron is in a less soluble ferric form (Fe+++) combined with OH. As pH increases above 9.5, more iron is found in the ferric form combined with OH. When sulfate levels in water increase above 200 ppm, iron increasingly complexes with sulfate to form FeSO4 rather than Fe (OH), and the palatability of water is likely to be reduced even more.
Manganese.
Manganese is one of the least toxic minerals and ruminants appear to tolerate large quantities of ingested manganese. One reason is the absorption of manganese is much lower than other minerals. The 2001 Nutrient Requirements of Dairy Cattle (NRC, 2001) put manganese availability from feedstuffs at 1%, however, Hurley and Keen (1987) reported it may be as high as 8%.
Manganese oxide is the most common form of manganese found in well waters. Although manganese is commonly included as one of the minerals to test for in the assessment of water quality, there are no reports of adverse effects in livestock from drinking water high in manganese (Agency for Toxic Substance and Disease Registry, 2000). The EPA (2004) water quality guidelines for human drinking water has a secondary standard maximum guideline of 0.05 ppm for manganese. Manganese does affect the taste of water for humans when levels exceed 0.05 ppm and it will form a black slime almost like crude oil in water troughs, cups and pipes if they are not routinely cleaned.
A study was conducted at study the University of Minnesota (Raeth-Knight et al. 2005) to determine if water high in manganese content affected the performance or water consumption of calves from 3 to 70 days of age. Thirty three (11 per treatment) calves were assigned at birth, to one of three water treatments, control, 0.25 ppm, or 0.75 ppm manganese. The control water contained 0.003 ppm manganese. The 0.25 and 0.75 ppm treatments were prepared by adding manganese carbonate to the control water. The three water treatments were used in the mixing of milk replacer and offered as free choice water to calves assigned to the respective treatments. Milk replacer was fed to 42 days of age and free choice water offered from 7 to 70 days.
Manganese level of water mixed with milk replacer or offered free choice had no affect on milk replacer, starter intake or growth of calves. Total DM intake (milk replacer plus starter) before weaning averaged 1.75 kg/day across all treatments. After weaning, DM intake (starter only) averaged 3.55 kg/day across all treatments. Average daily gain from birth to 70 days of age was 0.79, 0.75 and 0.79 kg for calves on control, 0.25 and 0.75 ppm manganese treatments, respectively. Water intake averaged 2.6, 2.7, and 2.7 liters/day during milk replacer feeding (day 3-42) and 10.8, 11.8 and 11.5 liters/day post-weaning (day 43-70) for control, 0.25, and 0.75 treatments, respectively.
Arsenic.
Arsenic is the 20th most abundant mineral in the earth's crust with an average concentration of 1.5 to 3 ppm. It is widely distributed geologically as a component of over 240 different minerals complexes. The natural form of arsenic is predominately arsenate occurring about 60% of the time, as sulfide or sulfur salt about 20% of the time and the remainder in many different forms. Most feeds contain less than 0.5 ppm arsenic and rarely exceed 1.0 ppm on a wet basis. In groundwater, most all of the arsenic is present as inorganic arsenic involving arsenate (+5) and arsenite (+3).
Water had not been considered a potential toxic source of arsenic until the 1980's when reports started to appear that as many as 20 countries around the world had water sources contaminated with arsenic. Arsenic concentrations as high as 500 ppb occur in some countries like Bangladesh. The United States EPA has listed arsenic as a human carcinogen and set a standard of 10 ppb in human drinking water beginning January 2006. A 1998-99 survey study in Minnesota found just under 10% of 869 wells in nine western Minnesota counties had arsenic levels above 50 ppb. Additional surveys in the upper Midwest have found arsenic concentrations occurring above the EPA limit of 10 ppb in 5 to 30% of sampled wells across.
Arsenic has been related to several forms of cancer in humans and chronic exposure to arsenic through drinking water has been linked to health effects such as nervous disorders, high blood pressure, diabetes and hyperkeratosis (Dowell, 2003). However, the effect of arsenic in drinking water on the health and/or performance of farm animals is relatively unknown.
Contribution of Water to Mineral Requirements
Table 2 shows water can be a major source of some macro-minerals for dairy cattle. Water can supply 0% (potassium) to over 100% (magnesium) of the daily mineral requirement assuming 100% of the mineral in water is available.
Table 2. Macro-mineral intake and impact of minerals in waters on the daily abrequirements of a dairy cow: water consumption - 108 liters; 38 kg milk product DM intake.
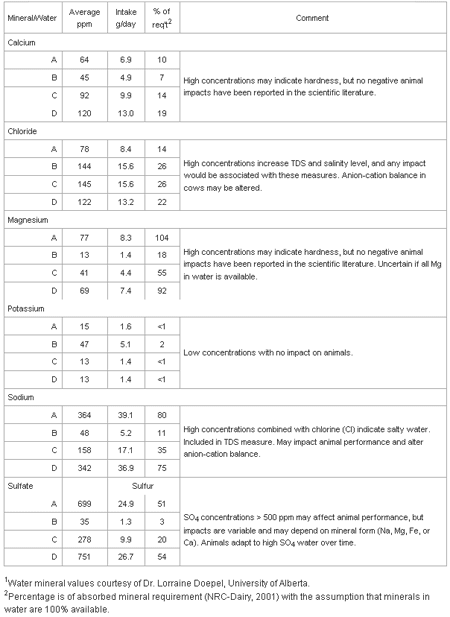
Studies have not been done to determine the availability of minerals from water, but the general assumption rightly or wrongly is dissolved minerals are nearly 100% available. An exception may be magnesium as it is absorbed across the rumen wall and availability is affected by potassium concentrations in the rumen and other factors. When water appears to be a major contributor to mineral requirements, probably over 20% using the assumption of 100% availability, it may indicate the quality of water is less than optimal and needs to be evaluated.
Table 3 shows the contribution of copper, iron and zinc in waters to the requirements of a dairy cow for these minerals.
Table 3. Micro-mineral intake and impact of minerals in waters on the daily abrequirements of a dairy cow: water consumption - 108 liters; 38 kg milk product DM intake.
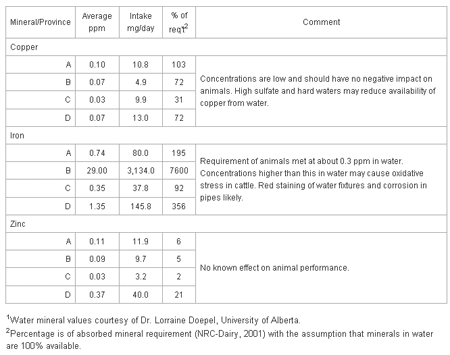
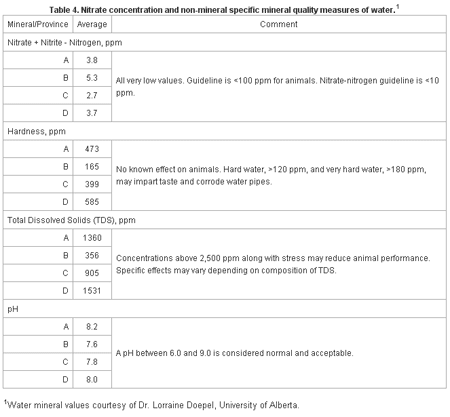
Table 4 shows other quality measures associated with waters. Hardness, pH and TDS measures reflect combinations or the sum of certain minerals and mineral groups found in water. They are useful in assessing water quality and the diagnosis of water related problems on farms, but generally lack specific detail as to problem causes, effects and why.
Table 4. Nitrate concentration and non-mineral specific mineral quality measures of waters.
Iron - Old wording
A high concentration (>0.3 ppm) of iron in drinking water is common. Human information suggests a high concentration of iron in water may reduce palatability and therefore consumption of water. Excess intakes of iron have been found to affect health through increasing reactive oxygen species (oxidative stress) which damages cell membranes and interrupts several biochemical reactions in the body. Oxidative stress in dairy cows has been related to increased incidences of mastitis, retained fetal membranes and a general decrease in immune function. The aesthetic problem with iron is in the presence of iron loving bacteria, reddish to black stains and slime form reducing water intake, staining porcelain and eventually restricting water flow through pipes.
The chemical form of iron in water is an important determinate of the bioavailability of iron and how oxidative reactive it may be. Most of the iron in water is soluble and found as ferrous (Fe++). However, the solubility and form of iron will change with pH and sulfate content of the water. At a pH of less than 7, more of the iron is in a less soluble ferric form (Fe+++) combined with OH. As pH increases above 9.5, more iron is found in the ferric form combined with OH. When sulfate levels in water increase above 200 ppm, iron increasingly complexes with sulfate to form FeSO4 rather than Fe (OH) and the palatability of water is likely to be reduced even more.
Take Home Message
- Water is the most essential of all nutrients required by dairy cattle.
- Cows consume water to meet their requirement. Limiting water intake by restricting access to or reducing consumption because of poor quality will decrease milk production. However, milk production and feed intake can not be stimulated by offering good quality water and enhancing water consumption above the amount needed to meet requirements.
- The mineral constituents found in water that have been shown by research to affect animal performance are: total dissolved solids (TDS), sodium chloride, sulfur (sulfate), and nitrate. Iron and manganese have been indicted in many water quality problems, but research directly linking iron and manganese to reduced water consumption and lowered milk production is lacking.
- Calcium, magnesium and water hardness are not believed to affect water intake or performance of animals.
by Jim Linn - Department of Animal Science, University of Minnesota
This article hasn't been commented yet.


Write a comment
* = required field